Learning the BAM format
Introduction
SAMtools provides various (sub)tools for manipulating alignments in the SAM/BAM format. The SAM (Sequence Alignment/Map) format (BAM is just the binary form of SAM) is currently the de facto standard for storing large nucleotide sequence alignments. If you are working with high-throughput sequencing data, at some point you will probably have to deal with SAM/BAM files, so familiarise yourself with them! For the latest information on SAMtools, please refer to the release notes.
The examples in this README use the ERR188273_chrX.bam BAM file
(stored in the eg folder) generated as per
https://github.com/davetang/rnaseq using the HISAT2 + StringTie2
RNA-seq pipeline. This README is generated using the create_readme.sh
script; if you want to generate this file yourself, please use this
Docker image
and the Makefile in this directory. For example:
# clone this repo
git clone https://github.com/davetang/learning_bam_file.git
cd learning_bam_file
docker pull davetang/r_build:4.1.2
docker run --rm -it -v $(pwd):/work davetang/r_build:4.1.2 /bin/bash
# inside the Docker container
make
Installing SAMtools
For installing SAMtools, I recommend using Conda and the Bioconda
samtools package. I also
recommend using
Miniconda instead of
Anaconda because Anaconda comes with a lot of tools/packages that you
will probably not use. I wrote a short introduction to
Conda
if you want to find learn more.
Once you have installed Miniconda, you can install SAMtools as follows:
conda install -c bioconda samtools
Otherwise you can download the source and compile it yourself; change
dir to the location you want samtools to be installed. samtools
will be installed in ${dir}/bin, so make sure this is in your $PATH.
#!/usr/bin/env bash
set -euo pipefail
ver=1.15
tool=samtools
url=https://github.com/samtools/${tool}/releases/download/${ver}/${tool}-${ver}.tar.bz2
dir=${HOME}/local
wget ${url}
tar xjf ${tool}-${ver}.tar.bz2
cd ${tool}-${ver}
./configure --prefix=${dir}
make && make install
cd ..
rm -rf ${tool}-${ver} ${tool}-${ver}.tar.bz2
>&2 echo Done
exit 0
Basic usage
If you run samtools on the terminal without any parameters or with
--help, all the available utilities are listed:
samtools --help
##
## Program: samtools (Tools for alignments in the SAM format)
## Version: 1.19.2 (using htslib 1.19.1)
##
## Usage: samtools <command> [options]
##
## Commands:
## -- Indexing
## dict create a sequence dictionary file
## faidx index/extract FASTA
## fqidx index/extract FASTQ
## index index alignment
##
## -- Editing
## calmd recalculate MD/NM tags and '=' bases
## fixmate fix mate information
## reheader replace BAM header
## targetcut cut fosmid regions (for fosmid pool only)
## addreplacerg adds or replaces RG tags
## markdup mark duplicates
## ampliconclip clip oligos from the end of reads
##
## -- File operations
## collate shuffle and group alignments by name
## cat concatenate BAMs
## consensus produce a consensus Pileup/FASTA/FASTQ
## merge merge sorted alignments
## mpileup multi-way pileup
## sort sort alignment file
## split splits a file by read group
## quickcheck quickly check if SAM/BAM/CRAM file appears intact
## fastq converts a BAM to a FASTQ
## fasta converts a BAM to a FASTA
## import Converts FASTA or FASTQ files to SAM/BAM/CRAM
## reference Generates a reference from aligned data
## reset Reverts aligner changes in reads
##
## -- Statistics
## bedcov read depth per BED region
## coverage alignment depth and percent coverage
## depth compute the depth
## flagstat simple stats
## idxstats BAM index stats
## cram-size list CRAM Content-ID and Data-Series sizes
## phase phase heterozygotes
## stats generate stats (former bamcheck)
## ampliconstats generate amplicon specific stats
##
## -- Viewing
## flags explain BAM flags
## head header viewer
## tview text alignment viewer
## view SAM<->BAM<->CRAM conversion
## depad convert padded BAM to unpadded BAM
## samples list the samples in a set of SAM/BAM/CRAM files
##
## -- Misc
## help [cmd] display this help message or help for [cmd]
## version detailed version information
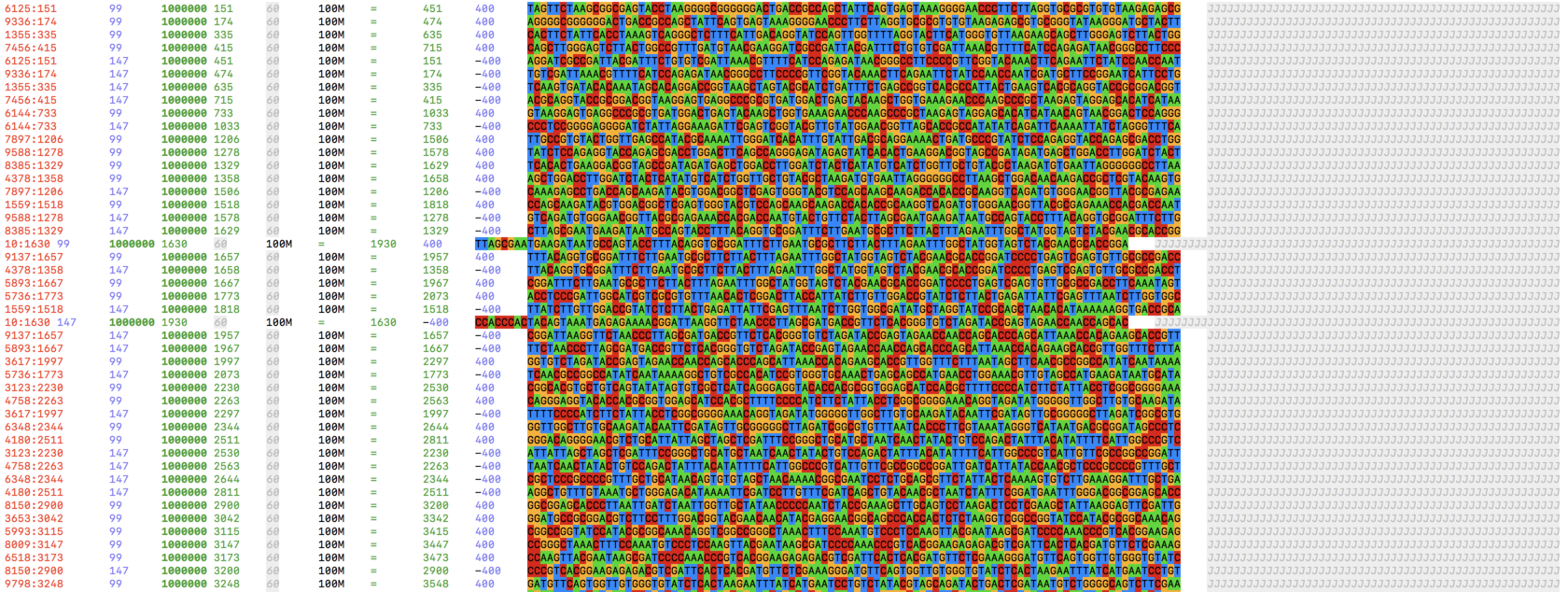
Viewing
Use bioSyntax to prettify your output.
samtools view aln.bam | sam-less
Converting a SAM file to a BAM file
A BAM file is just a SAM file but stored in binary format; you should always convert your SAM files into BAM format since they are smaller in size and are faster to manipulate.
I don’t have a SAM file in the example folder, so let’s create one and
check out the first ten lines. Note: remember to use -h to ensure the
SAM file contains the sequence header information. Generally, I
recommend storing only sorted BAM files as they use even less disk space
and are faster to process.
samtools view -h eg/ERR188273_chrX.bam > eg/ERR188273_chrX.sam
Notice that the SAM file is much larger than the BAM file.
Size of SAM file.
ls -lh eg/ERR188273_chrX.sam
## -rw-r--r-- 1 root root 321M Mar 25 07:28 eg/ERR188273_chrX.sam
Size of BAM file.
ls -lh eg/ERR188273_chrX.bam
## -rw-r--r-- 1 root root 67M Mar 25 07:27 eg/ERR188273_chrX.bam
We can use head to view a SAM file.
head eg/ERR188273_chrX.sam
## @HD VN:1.0 SO:coordinate
## @SQ SN:chrX LN:156040895
## @PG ID:hisat2 PN:hisat2 VN:2.2.0 CL:"/Users/dtang/github/rnaseq/hisat2/../src/hisat2-2.2.0/hisat2-align-s --wrapper basic-0 --dta -p 4 -x ../raw/chrX_data/indexes/chrX_tran -1 /tmp/4195.inpipe1 -2 /tmp/4195.inpipe2"
## @PG ID:samtools PN:samtools PP:hisat2 VN:1.19.2 CL:samtools view -h eg/ERR188273_chrX.bam
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
## ERR188273.4711308 329 chrX 233717 0 5S70M = 233717 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.14904746 99 chrX 251271 60 75M = 251317 121 GAAAAATGGGCCCAGGGGACCGGCGCTCAGCATACAGAGGACCCGCGCCGGCACCTGCCTCTGAGTTCCCTTAGT @@<DDDDDFB>HHEGIIGAGIIIBGIIG@FECH<F@GIIFAE=?BCBBCBBB5@<?CBBCCCCAACDCCCCCCCC AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:-2 YT:Z:CP NH:i:1
## ERR188273.14904746 147 chrX 251317 60 75M = 251271 -121 GCCGGCCCCTGCCTCTGAGTTCCCTTAGTACTTATTGATCATTATCGGGGAGAGGGGGATGTGGCAGGACAATAG #######B?DAHC@EGIIGGEHHGC@GFBFCEGFCIGG@EG@@H<JIEHEF@IGEHGHIIHFGHDDFDDDDD?<B AS:i:-2 ZS:i:-7 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:6A68 YS:i:0 YT:Z:CP NH:i:1
## ERR188273.5849805 163 chrX 265951 1 75M = 266022 146 CGGGTTCACGCCATTCTCCTGCCTCAGCCTCCCGAGTAGCTGGGACTACAGGCGCCCGCCACCACGCCCGGCTAA @CCFDFFFHHHHHJJJJJJFJJJJJIJIJJJJJJJJGHIJJJJJEHIJIJGIIJJJHHFFDDEDDDDDDDDDDDD AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:0 YT:Z:CP NH:i:2
The lines starting with an “@” symbol contains the header information.
The @SQ tag is the reference sequence dictionary; SN refers to the
reference sequence name and LN refers to the reference sequence length.
If you don’t see lines starting with the “@” symbol, the header
information is probably missing. You can generate this information again
by running the command below, where ref.fa is the reference FASTA file
used to map the reads.
samtools view -bT sequence/ref.fa aln.sam > aln.bam
If the header information is available, we can convert a SAM file into
BAM by using samtools view -b. In newer versions of SAMtools, the
input format is auto-detected, so we no longer need the -S parameter.
samtools view -b eg/ERR188273_chrX.sam > eg/my.bam
Converting a BAM file to a CRAM file
The CRAM format is even more compact. Use samtools view with the -T
and -C arguments to convert a BAM file into CRAM.
samtools view -T genome/chrX.fa -C -o eg/ERR188273_chrX.cram eg/ERR188273_chrX.bam
ls -lh eg/ERR188273_chrX.[sbcr]*am
## -rw-r--r-- 1 root root 67M Mar 25 07:27 eg/ERR188273_chrX.bam
## -rw-r--r-- 1 root root 40M Mar 25 07:28 eg/ERR188273_chrX.cram
## -rw-r--r-- 1 root root 321M Mar 25 07:28 eg/ERR188273_chrX.sam
You can use samtools view to view a CRAM file just as you would for a
BAM file.
samtools view eg/ERR188273_chrX.cram | head
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YT:Z:UP NH:i:2 MD:Z:70 NM:i:0
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
## ERR188273.4711308 329 chrX 233717 0 5S70M = 233717 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YT:Z:UP NH:i:2 MD:Z:70 NM:i:0
## ERR188273.14904746 99 chrX 251271 60 75M = 251317 121 GAAAAATGGGCCCAGGGGACCGGCGCTCAGCATACAGAGGACCCGCGCCGGCACCTGCCTCTGAGTTCCCTTAGT @@<DDDDDFB>HHEGIIGAGIIIBGIIG@FECH<F@GIIFAE=?BCBBCBBB5@<?CBBCCCCAACDCCCCCCCC AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YS:i:-2 YT:Z:CP NH:i:1 MD:Z:75 NM:i:0
## ERR188273.14904746 147 chrX 251317 60 75M = 251271 -121 GCCGGCCCCTGCCTCTGAGTTCCCTTAGTACTTATTGATCATTATCGGGGAGAGGGGGATGTGGCAGGACAATAG #######B?DAHC@EGIIGGEHHGC@GFBFCEGFCIGG@EG@@H<JIEHEF@IGEHGHIIHFGHDDFDDDDD?<B AS:i:-2 ZS:i:-7 XN:i:0 XM:i:1 XO:i:0 XG:i:0 YS:i:0 YT:Z:CP NH:i:1 MD:Z:6A68 NM:i:1
## ERR188273.5849805 163 chrX 265951 1 75M = 266022 146 CGGGTTCACGCCATTCTCCTGCCTCAGCCTCCCGAGTAGCTGGGACTACAGGCGCCCGCCACCACGCCCGGCTAA @CCFDFFFHHHHHJJJJJJFJJJJJIJIJJJJJJJJGHIJJJJJEHIJIJGIIJJJHHFFDDEDDDDDDDDDDDD AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YS:i:0 YT:Z:CP NH:i:2 MD:Z:75 NM:i:0
## ERR188273.1232356 369 chrX 265984 1 75M = 118343251 0 GAGTAGCTGGGACTACAGGCGCCCGCCACCACGCCCGGCTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCAC @@CA@B>DC>>+@::8-755-BBBFDDEHHBGGEGHEEIJIIGIJJIGEIIIJJJIIJJIGGHHHGGFFFFF@@C AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YT:Z:UP NH:i:10 MD:Z:75 NM:i:0
## ERR188273.5927795 385 chrX 265991 1 75M = 114048277 0 TGGGACTACAGGCGCCCGCCACCACGCCCGGCTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGTTA =?BB??BD?FBHHBEAE@CDGG@HH=FA@GEGE;FGACCHBE6?A=ACE9)7@DCE>>5'3=338:;:>2<AA?: AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YT:Z:UP NH:i:10 MD:Z:75 NM:i:0
## ERR188273.5849805 83 chrX 266022 1 75M = 265951 -146 CTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGTTAGCCAGGATGGTGTCGATCTCCTGACCTCGTG DDDDDDDEEEEEDBFEDGHHHHHHJHIJJIGIGHFBJJIHGJJIIJJJJJJJJIGIJJJJJJHHHHHDFFFFCCB AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YS:i:0 YT:Z:CP NH:i:2 MD:Z:75 NM:i:0
## ERR188273.13655123 113 chrX 266022 1 75M = 118343234 0 CTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGTTAGCCAGGATGGTGTCGATCTCCTGACCTCGTG AACBBBACCCC>;3?BCFFEEHHHEEGIGGHAGFBBHFBHHEHCG@<@ABG??@@?BB9GBGAFFD<<DDAD@@@ AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 YT:Z:UP NH:i:2 MD:Z:75 NM:i:0
I have an old blog post on the CRAM format.
Sorting a SAM/BAM file
Many downstream tools require sorted BAM files and since they are slightly more compact than unsorted BAM files, you should always sorted BAM files. In SAMtools version 1.3 or newer, you can directly generate a sorted BAM file from a SAM file.
samtools sort eg/ERR188273_chrX.sam -o eg/sorted.bam
ls -l eg/ERR188273_chrX.bam
ls -l eg/sorted.bam
## -rw-r--r-- 1 root root 69983526 Mar 25 07:27 eg/ERR188273_chrX.bam
## -rw-r--r-- 1 root root 69983599 Mar 25 07:29 eg/sorted.bam
You should use use additional threads (if they are available) to speed
up sorting; to use four threads, use -@ 4.
Time taken using one thread (default).
time samtools sort eg/ERR188273_chrX.sam -o eg/sorted.bam
##
## real 0m8.748s
## user 0m8.458s
## sys 0m0.244s
Time taken using four threads.
time samtools sort -@ 4 eg/ERR188273_chrX.sam -o eg/sorted.bam
## [bam_sort_core] merging from 0 files and 4 in-memory blocks...
##
## real 0m2.896s
## user 0m10.513s
## sys 0m0.484s
Many of the SAMtools subtools can use additional threads, so make use of them if you have the resources!
Creating a BAM index file
Various tools require BAM index files, such as IGV, which is a tool that can be used for visualising BAM files.
samtools index eg/ERR188273_chrX.bam
Adding read groups
Some tools like GATK and Picard require read
groups
(RG). You can add or replace read groups using samtools addreplacerg.
samtools addreplacerg -r "@RG\tID:ERR188273\tSM:ERR188273\tPL:illumina" -o eg/ERR188273_chrX_rg.bam eg/ERR188273_chrX.bam
samtools head eg/ERR188273_chrX_rg.bam
## @HD VN:1.0 SO:coordinate
## @SQ SN:chrX LN:156040895
## @PG ID:hisat2 PN:hisat2 VN:2.2.0 CL:"/Users/dtang/github/rnaseq/hisat2/../src/hisat2-2.2.0/hisat2-align-s --wrapper basic-0 --dta -p 4 -x ../raw/chrX_data/indexes/chrX_tran -1 /tmp/4195.inpipe1 -2 /tmp/4195.inpipe2"
## @PG ID:samtools PN:samtools PP:hisat2 VN:1.19.2 CL:samtools addreplacerg -r @RG\tID:ERR188273\tSM:ERR188273\tPL:illumina -o eg/ERR188273_chrX_rg.bam eg/ERR188273_chrX.bam
## @RG ID:ERR188273 SM:ERR188273 PL:illumina
If you want to replace existing read groups, just use the same command.
samtools addreplacerg -r "@RG\tID:ERR188273_2\tSM:ERR188273_2\tPL:illumina_2" -o eg/ERR188273_chrX_rg2.bam eg/ERR188273_chrX_rg.bam
samtools head eg/ERR188273_chrX_rg2.bam
## @HD VN:1.0 SO:coordinate
## @SQ SN:chrX LN:156040895
## @PG ID:hisat2 PN:hisat2 VN:2.2.0 CL:"/Users/dtang/github/rnaseq/hisat2/../src/hisat2-2.2.0/hisat2-align-s --wrapper basic-0 --dta -p 4 -x ../raw/chrX_data/indexes/chrX_tran -1 /tmp/4195.inpipe1 -2 /tmp/4195.inpipe2"
## @PG ID:samtools PN:samtools PP:hisat2 VN:1.19.2 CL:samtools addreplacerg -r @RG\tID:ERR188273\tSM:ERR188273\tPL:illumina -o eg/ERR188273_chrX_rg.bam eg/ERR188273_chrX.bam
## @PG ID:samtools.1 PN:samtools PP:samtools VN:1.19.2 CL:samtools addreplacerg -r @RG\tID:ERR188273_2\tSM:ERR188273_2\tPL:illumina_2 -o eg/ERR188273_chrX_rg2.bam eg/ERR188273_chrX_rg.bam
## @RG ID:ERR188273_2 SM:ERR188273_2 PL:illumina_2
Popular alignment tools such as BWA MEM and STAR can add read groups;
use the -R and --outSAMattrRGline parameters for the respective
tool.
bwa mem \
-M \
-t ${thread} \
-R "@RG\tID:${sample_name}\tSM:${sample}\tPL:${platform}" \
${fasta} \
${fastq1} \
${fastq2} |
samtools sort -@ ${thread} -O BAM |\
tee ${sample_name}.bam |\
samtools index - ${sample_name}.bam.bai
STAR \
--runMode alignReads \
--genomeDir ${star_index} \
--readFilesIn ${fastq1} ${fastq2} \
--readFilesCommand "gunzip -c" \
--outFileNamePrefix ${prefix}. \
--outSAMtype BAM Unsorted \
--twopassMode Basic \
--outSAMattrRGline ID:${id} PL:Illumina PU:${pu} LB:${lb} PI:0 SM:${sm} \
--outSAMattributes NH HI AS nM NM ch \
--runThreadN ${num_threads}
Interpreting the BAM flags
The second column in a SAM/BAM file is the flag column; use the flags
subcommand to understand specific flags. They may seem confusing at
first but the encoding allows details about a read to be stored by just
using a few digits. The trick is to convert the numerical digit into
binary, and then use the table to interpret the binary numbers, where 1
= true and 0 = false. I wrote a blog post on BAM flags at
http://davetang.org/muse/2014/03/06/understanding-bam-flags/.
samtools flags
## About: Convert between textual and numeric flag representation
## Usage: samtools flags FLAGS...
##
## Each FLAGS argument is either an INT (in decimal/hexadecimal/octal) representing
## a combination of the following numeric flag values, or a comma-separated string
## NAME,...,NAME representing a combination of the following flag names:
##
## 0x1 1 PAIRED paired-end / multiple-segment sequencing technology
## 0x2 2 PROPER_PAIR each segment properly aligned according to aligner
## 0x4 4 UNMAP segment unmapped
## 0x8 8 MUNMAP next segment in the template unmapped
## 0x10 16 REVERSE SEQ is reverse complemented
## 0x20 32 MREVERSE SEQ of next segment in template is rev.complemented
## 0x40 64 READ1 the first segment in the template
## 0x80 128 READ2 the last segment in the template
## 0x100 256 SECONDARY secondary alignment
## 0x200 512 QCFAIL not passing quality controls or other filters
## 0x400 1024 DUP PCR or optical duplicate
## 0x800 2048 SUPPLEMENTARY supplementary alignment
Find out about a 73 flag.
samtools flags 73
## 0x49 73 PAIRED,MUNMAP,READ1
Proper pair
Reads that are properly paired are mapped within an expected distance
with each other and with one pair in the reverse complement orientation.
The script generate_random_seq.pl can generate reads that originate
from different references and are thus discordant and not properly
paired (as well as properly paired reads). In the example below, 10% of
reads are not properly paired (set with -d 0.1).
script/generate_random_seq.pl 30 10000 1984 > test_ref.fa
script/random_paired_end.pl -f test_ref.fa -l 100 -n 10000 -m 300 -d 0.1
bwa index test_ref.fa 2> /dev/null
bwa mem test_ref.fa l100_n10000_d300_1984_1.fq.gz l100_n10000_d300_1984_2.fq.gz > aln.sam 2> /dev/null
samtools flagstat will indicate that some reads (about 10%) mapped to
different chromosomes.
samtools flagstat aln.sam
## 20000 + 0 in total (QC-passed reads + QC-failed reads)
## 20000 + 0 primary
## 0 + 0 secondary
## 0 + 0 supplementary
## 0 + 0 duplicates
## 0 + 0 primary duplicates
## 20000 + 0 mapped (100.00% : N/A)
## 20000 + 0 primary mapped (100.00% : N/A)
## 20000 + 0 paired in sequencing
## 10000 + 0 read1
## 10000 + 0 read2
## 18012 + 0 properly paired (90.06% : N/A)
## 20000 + 0 with itself and mate mapped
## 0 + 0 singletons (0.00% : N/A)
## 1988 + 0 with mate mapped to a different chr
## 1988 + 0 with mate mapped to a different chr (mapQ>=5)
Flag of a proper pair.
samtools flag $(samtools view -f 2 aln.sam | head -1 | cut -f2)
## 0x63 99 PAIRED,PROPER_PAIR,MREVERSE,READ1
Flag of a pair (that is not a proper pair).
samtools flag $(samtools view -F 2 aln.sam | head -1 | cut -f2)
## 0x61 97 PAIRED,MREVERSE,READ1
Filtering unmapped reads
Use -F 4 to filter out unmapped reads.
samtools view -F 4 -b eg/ERR188273_chrX.bam > eg/ERR188273_chrX.mapped.bam
Use -f 4 to keep only unmapped reads.
samtools view -f 4 -b eg/ERR188273_chrX.bam > eg/ERR188273_chrX.unmapped.bam
We can use the flags subcommand to confirm that a value of four
represents an unmapped read.
samtools flags 4
## 0x4 4 UNMAP
Extracting entries mapping to a specific loci
Use samtools view and the ref:start-end syntax to extract reads
mapping within a specific genomic loci; this requires a BAM index file.
samtools view eg/ERR188273_chrX.bam chrX:20000-30000
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
Note that this takes into account the mapping of the entire read and not just the starting position. For example, if you specified chrX:20000-30000, a 75 bp long read that starts its mapping from position 19999 will also be returned. In addition, you can save the output as another BAM file if you want.
samtools view -b eg/ERR188273_chrX.bam chrX:20000-30000 > eg/ERR188273_chrX_20000_30000.bam
If you want reads mapped to a single reference (e.g. chromosome), just
specify the ref and leave out the start and end values.
samtools view eg/ERR188273_chrX.bam chrX | head
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
## ERR188273.4711308 329 chrX 233717 0 5S70M = 233717 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.14904746 99 chrX 251271 60 75M = 251317 121 GAAAAATGGGCCCAGGGGACCGGCGCTCAGCATACAGAGGACCCGCGCCGGCACCTGCCTCTGAGTTCCCTTAGT @@<DDDDDFB>HHEGIIGAGIIIBGIIG@FECH<F@GIIFAE=?BCBBCBBB5@<?CBBCCCCAACDCCCCCCCC AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:-2 YT:Z:CP NH:i:1
## ERR188273.14904746 147 chrX 251317 60 75M = 251271 -121 GCCGGCCCCTGCCTCTGAGTTCCCTTAGTACTTATTGATCATTATCGGGGAGAGGGGGATGTGGCAGGACAATAG #######B?DAHC@EGIIGGEHHGC@GFBFCEGFCIGG@EG@@H<JIEHEF@IGEHGHIIHFGHDDFDDDDD?<B AS:i:-2 ZS:i:-7 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:6A68 YS:i:0 YT:Z:CP NH:i:1
## ERR188273.5849805 163 chrX 265951 1 75M = 266022 146 CGGGTTCACGCCATTCTCCTGCCTCAGCCTCCCGAGTAGCTGGGACTACAGGCGCCCGCCACCACGCCCGGCTAA @CCFDFFFHHHHHJJJJJJFJJJJJIJIJJJJJJJJGHIJJJJJEHIJIJGIIJJJHHFFDDEDDDDDDDDDDDD AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:0 YT:Z:CP NH:i:2
## ERR188273.1232356 369 chrX 265984 1 75M = 118343251 0 GAGTAGCTGGGACTACAGGCGCCCGCCACCACGCCCGGCTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCAC @@CA@B>DC>>+@::8-755-BBBFDDEHHBGGEGHEEIJIIGIJJIGEIIIJJJIIJJIGGHHHGGFFFFF@@C AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YT:Z:UP NH:i:10
## ERR188273.5927795 385 chrX 265991 1 75M = 114048277 0 TGGGACTACAGGCGCCCGCCACCACGCCCGGCTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGTTA =?BB??BD?FBHHBEAE@CDGG@HH=FA@GEGE;FGACCHBE6?A=ACE9)7@DCE>>5'3=338:;:>2<AA?: AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YT:Z:UP NH:i:10
## ERR188273.5849805 83 chrX 266022 1 75M = 265951 -146 CTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGTTAGCCAGGATGGTGTCGATCTCCTGACCTCGTG DDDDDDDEEEEEDBFEDGHHHHHHJHIJJIGIGHFBJJIHGJJIIJJJJJJJJIGIJJJJJJHHHHHDFFFFCCB AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:0 YT:Z:CP NH:i:2
## ERR188273.13655123 113 chrX 266022 1 75M = 118343234 0 CTAATTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGTTAGCCAGGATGGTGTCGATCTCCTGACCTCGTG AACBBBACCCC>;3?BCFFEEHHHEEGIGGHAGFBBHFBHHEHCG@<@ABG??@@?BB9GBGAFFD<<DDAD@@@ AS:i:0 ZS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YT:Z:UP NH:i:2
You can also use a BED file, with several entries, to extract reads of interest.
cat eg/my.bed
samtools view -L eg/my.bed eg/ERR188273_chrX.bam
## chrX 20000 30000
## chrX 233000 260000
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
## ERR188273.4711308 329 chrX 233717 0 5S70M = 233717 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.14904746 99 chrX 251271 60 75M = 251317 121 GAAAAATGGGCCCAGGGGACCGGCGCTCAGCATACAGAGGACCCGCGCCGGCACCTGCCTCTGAGTTCCCTTAGT @@<DDDDDFB>HHEGIIGAGIIIBGIIG@FECH<F@GIIFAE=?BCBBCBBB5@<?CBBCCCCAACDCCCCCCCC AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:-2 YT:Z:CP NH:i:1
## ERR188273.14904746 147 chrX 251317 60 75M = 251271 -121 GCCGGCCCCTGCCTCTGAGTTCCCTTAGTACTTATTGATCATTATCGGGGAGAGGGGGATGTGGCAGGACAATAG #######B?DAHC@EGIIGGEHHGC@GFBFCEGFCIGG@EG@@H<JIEHEF@IGEHGHIIHFGHDDFDDDDD?<B AS:i:-2 ZS:i:-7 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:6A68 YS:i:0 YT:Z:CP NH:i:1
Extracting only the first read from paired end BAM files
Sometimes you only want the first pair of a mate. 0x0040 is hexadecimal for 64 (i.e. 16 * 4), which is binary for 1000000, corresponding to the read in the first read pair.
samtools view -b -f 0x0040 eg/ERR188273_chrX.bam > eg/first.bam
Once again, you can use flags to verify this (it also accepts
hexadecimal input).
samtools flags 0x0040
## 0x40 64 READ1
Stats
For simple statistics use samtools flagstat.
samtools flagstat eg/ERR188273_chrX.bam
## 1176360 + 0 in total (QC-passed reads + QC-failed reads)
## 1160084 + 0 primary
## 16276 + 0 secondary
## 0 + 0 supplementary
## 0 + 0 duplicates
## 0 + 0 primary duplicates
## 1126961 + 0 mapped (95.80% : N/A)
## 1110685 + 0 primary mapped (95.74% : N/A)
## 1160084 + 0 paired in sequencing
## 580042 + 0 read1
## 580042 + 0 read2
## 1060858 + 0 properly paired (91.45% : N/A)
## 1065618 + 0 with itself and mate mapped
## 45067 + 0 singletons (3.88% : N/A)
## 0 + 0 with mate mapped to a different chr
## 0 + 0 with mate mapped to a different chr (mapQ>=5)
For more stats, use samtools stats.
samtools stats eg/ERR188273_chrX.bam | grep ^SN
## SN raw total sequences: 1160084 # excluding supplementary and secondary reads
## SN filtered sequences: 0
## SN sequences: 1160084
## SN is sorted: 1
## SN 1st fragments: 580042
## SN last fragments: 580042
## SN reads mapped: 1110685
## SN reads mapped and paired: 1065618 # paired-end technology bit set + both mates mapped
## SN reads unmapped: 49399
## SN reads properly paired: 1060858 # proper-pair bit set
## SN reads paired: 1160084 # paired-end technology bit set
## SN reads duplicated: 0 # PCR or optical duplicate bit set
## SN reads MQ0: 905 # mapped and MQ=0
## SN reads QC failed: 0
## SN non-primary alignments: 16276
## SN supplementary alignments: 0
## SN total length: 87006300 # ignores clipping
## SN total first fragment length: 43503150 # ignores clipping
## SN total last fragment length: 43503150 # ignores clipping
## SN bases mapped: 83301375 # ignores clipping
## SN bases mapped (cigar): 83064942 # more accurate
## SN bases trimmed: 0
## SN bases duplicated: 0
## SN mismatches: 423271 # from NM fields
## SN error rate: 5.095663e-03 # mismatches / bases mapped (cigar)
## SN average length: 75
## SN average first fragment length: 75
## SN average last fragment length: 75
## SN maximum length: 75
## SN maximum first fragment length: 75
## SN maximum last fragment length: 75
## SN average quality: 36.0
## SN insert size average: 182.7
## SN insert size standard deviation: 176.0
## SN inward oriented pairs: 530763
## SN outward oriented pairs: 1042
## SN pairs with other orientation: 1004
## SN pairs on different chromosomes: 0
## SN percentage of properly paired reads (%): 91.4
samtools calmd/fillmd
The calmd or fillmd tool is useful for visualising mismatches and
insertions in an alignment of a read to a reference genome. The -e
argument changes identical bases between the read and reference into
=.
samtools view -b eg/ERR188273_chrX.bam | samtools fillmd -e - genome/chrX.fa > eg/ERR188273_chrX_fillmd.bam
head eg/ERR188273_chrX_fillmd.bam
## @HD VN:1.0 SO:coordinate
## @SQ SN:chrX LN:156040895
## @PG ID:hisat2 PN:hisat2 VN:2.2.0 CL:"/Users/dtang/github/rnaseq/hisat2/../src/hisat2-2.2.0/hisat2-align-s --wrapper basic-0 --dta -p 4 -x ../raw/chrX_data/indexes/chrX_tran -1 /tmp/4195.inpipe1 -2 /tmp/4195.inpipe2"
## @PG ID:samtools PN:samtools PP:hisat2 VN:1.19.2 CL:samtools view -b eg/ERR188273_chrX.bam
## @PG ID:samtools.1 PN:samtools PP:samtools VN:1.19.2 CL:samtools fillmd -e - genome/chrX.fa
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGT====================================================================== @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
## ERR188273.4711308 329 chrX 233717 0 5S70M = 233717 0 CGGGT====================================================================== @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.14904746 99 chrX 251271 60 75M = 251317 121 =========================================================================== @@<DDDDDFB>HHEGIIGAGIIIBGIIG@FECH<F@GIIFAE=?BCBBCBBB5@<?CBBCCCCAACDCCCCCCCC AS:i:0 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:75 YS:i:-2 YT:Z:CP NH:i:1
## ERR188273.14904746 147 chrX 251317 60 75M = 251271 -121 ======C==================================================================== #######B?DAHC@EGIIGGEHHGC@GFBFCEGFCIGG@EG@@H<JIEHEF@IGEHGHIIHFGHDDFDDDDD?<B AS:i:-2 ZS:i:-7 XN:i:0 XM:i:1 XO:i:0 XG:i:0 NM:i:1 MD:Z:6A68 YS:i:0 YT:Z:CP NH:i:1
Creating FASTQ files from a BAM file
Use the fastq tool to create FASTQ files from a BAM file. For
paired-end reads, use -1 and -2 to create separate FASTA files.
samtools fastq -1 eg/ERR188273_chrX_1.fq -2 eg/ERR188273_chrX_2.fq eg/ERR188273_chrX.bam
head eg/ERR188273_chrX_1.fq
## [M::bam2fq_mainloop] discarded 0 singletons
## [M::bam2fq_mainloop] processed 1160084 reads
## @ERR188273.4711308
## CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA
## +
## @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@
## @ERR188273.14904746
## GAAAAATGGGCCCAGGGGACCGGCGCTCAGCATACAGAGGACCCGCGCCGGCACCTGCCTCTGAGTTCCCTTAGT
## +
## @@<DDDDDFB>HHEGIIGAGIIIBGIIG@FECH<F@GIIFAE=?BCBBCBBB5@<?CBBCCCCAACDCCCCCCCC
## @ERR188273.5849805
## CACGAGGTCAGGAGATCGACACCATCCTGGCTAACACGGTGAAACCCCGTCTCTACTAAAAATACAAAAAATTAG
Random subsampling of BAM file
The SAMtools view -s parameter allows you to randomly sub-sample a BAM
file. Using -s 0.5 will create a new BAM file with a random half of
all mapped reads; unmapped reads are not sampled.
samtools view -s 0.5 -b eg/ERR188273_chrX.bam > eg/ERR188273_chrX_rand.bam
Count number of reads
Use samtools idxstats to print stats on a BAM file; this requires an
index file which is created by running samtools index. The output of
idxstats is a file with four tab-delimited columns:
- Reference name
- Sequence length of reference
- Number of mapped reads
- Number of unmapped reads
samtools idxstats eg/ERR188273_chrX.bam
## chrX 156040895 1126961 45067
## * 0 0 4332
We can use this with awk to calculate:
The number of mapped reads by summing the third column.
samtools idxstats eg/ERR188273_chrX.bam | awk '{s+=$3} END {print s}'
## 1126961
The number of reads, which is the sum of mapped and unmapped reads.
samtools idxstats eg/ERR188273_chrX.bam | awk '{s+=$3+$4} END {print s}'
## 1176360
Obtaining genomic sequence
Use faidx to fetch genomic sequence; coordinates are 1-based.
We need to first index the reference FASTA file that was used to map the reads.
samtools faidx genome/chrX.fa
Now we can obtain the sequence.
samtools faidx genome/chrX.fa chrX:300000-300100
## >chrX:300000-300100
## ctgagatcgtgccactgcactccagcctgggcgacagagcgagactccatctcaaaaaaa
## aaaaaaaaaaaaaagaTggggtctctctatgttggccaggt
Comparing BAM files
The output from mpileup can be used to compare BAM files. The commands
below generates alignments using bwa and minimap2.
len=100
n=10000
m=300
script/generate_random_seq.pl 30 1000000 1984 > test_ref.fa
script/random_paired_end.pl -f test_ref.fa -l ${len} -n ${n} -m ${m}
bwa index test_ref.fa 2> /dev/null
bwa mem test_ref.fa l${len}_n${n}_d${m}_1984_1.fq.gz l${len}_n${n}_d${m}_1984_2.fq.gz 2> /dev/null | samtools sort - -o aln_bwa.bam
minimap2 -ax sr test_ref.fa l${len}_n${n}_d${m}_1984_1.fq.gz l${len}_n${n}_d${m}_1984_2.fq.gz 2> /dev/null | samtools sort - -o aln_mm.bam
The BAM files can be used with mpileup to compare the depths.
samtools mpileup -s -f test_ref.fa aln_bwa.bam aln_mm.bam | head -20
## [mpileup] 2 samples in 2 input files
## 1 8238 G 1 ^]. > ] 1 ^]. > ]
## 1 8239 G 1 . > ] 1 . > ]
## 1 8240 A 1 . J ] 1 . J ]
## 1 8241 C 1 . J ] 1 . J ]
## 1 8242 A 1 . J ] 1 . J ]
## 1 8243 C 1 . J ] 1 . J ]
## 1 8244 T 1 . J ] 1 . J ]
## 1 8245 G 1 . J ] 1 . J ]
## 1 8246 C 1 . J ] 1 . J ]
## 1 8247 G 1 . J ] 1 . J ]
## 1 8248 A 1 . J ] 1 . J ]
## 1 8249 C 1 . J ] 1 . J ]
## 1 8250 A 1 . J ] 1 . J ]
## 1 8251 G 1 . J ] 1 . J ]
## 1 8252 T 1 . J ] 1 . J ]
## 1 8253 G 1 . J ] 1 . J ]
## 1 8254 A 1 . J ] 1 . J ]
## 1 8255 G 1 . J ] 1 . J ]
## 1 8256 G 1 . J ] 1 . J ]
## 1 8257 G 1 . J ] 1 . J ]
Another approach is to use
deepTools and the
bamCompare
command. The bigWig output file shows the ratio of reads between b1
and b2 in 50 bp (default) windows.
Converting reference names
One of the most annoying bioinformatics problems is the use of different
chromosome names, e.g. chr1 vs 1, in different references even when the
sequences are identical. The GRCh38 reference downloaded from Ensembl
has chromosome names without the chr:
>1 dna:chromosome chromosome:GRCh38:1:1:248956422:1 REF
Whereas the reference names from UCSC has the chr:
>chr1 AC:CM000663.2 gi:568336023 LN:248956422 rl:Chromosome M5:6aef897c3d6ff0c78aff06ac189178dd AS:GRCh38
Luckily you can change the reference names using samtools reheader but
just make sure your reference sequences are actually identical.
samtools view eg/ERR188273_chrX.bam | head -2
## ERR188273.4711308 73 chrX 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 chrX 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
View header
samtools view -H eg/ERR188273_chrX.bam
## @HD VN:1.0 SO:coordinate
## @SQ SN:chrX LN:156040895
## @PG ID:hisat2 PN:hisat2 VN:2.2.0 CL:"/Users/dtang/github/rnaseq/hisat2/../src/hisat2-2.2.0/hisat2-align-s --wrapper basic-0 --dta -p 4 -x ../raw/chrX_data/indexes/chrX_tran -1 /tmp/4195.inpipe1 -2 /tmp/4195.inpipe2"
## @PG ID:samtools PN:samtools PP:hisat2 VN:1.19.2 CL:samtools view -H eg/ERR188273_chrX.bam
Substitute header with new name.
samtools view -H eg/ERR188273_chrX.bam | sed 's/SN:chrX/SN:X/' > eg/my_header
Save bam file with new ref and check it out.
samtools reheader eg/my_header eg/ERR188273_chrX.bam > eg/ERR188273_X.bam
samtools view eg/ERR188273_X.bam | head -2
## ERR188273.4711308 73 X 21649 0 5S70M = 21649 0 CGGGTGATCACGAGGTCAGGAGATCAAGACCATCCTGGCCAACACAGTGAAACCCCATCTCTACTAAAAATACAA @@@F=DDFFHGHBHIFFHIGGIFGEGHFHIGIGIFIIIGIGIGGDHIIGIIC@>DGHCHHHGHHFFFFFDEACC@ AS:i:-5 ZS:i:-5 XN:i:0 XM:i:0 XO:i:0 XG:i:0 NM:i:0 MD:Z:70 YT:Z:UP NH:i:2
## ERR188273.4711308 133 X 21649 0 * = 21649 0 CTACAGGTGCCCGCCACCATGCCCAGCTAATTTTTTTTGTATTTTTAGTAGAGATGGGGTTTCACTGTGTTGGCC CB@FDFFFHHGFHIJJJJIIIIIIIGGGIJGIIJJJJJJFFHIIIIGECHEHHGGHHFF?AACCDDDDDDDDBCD YT:Z:UP
Coverage
Coverage can mean the:
- average depth of each covered base
- percentage of bases covered
samtools depth and samtools mpileup can be used to indicate the
depth of each covered base (and used to calculate the average depth.
samtools coverage will provide both the average depth and percentage
of bases covered per chromosome/reference sequence.
samtools depth will return three columns: reference, position, and
coverage.
samtools depth -@ 4 eg/ERR188273_chrX.bam > ERR188273_depth.tsv
head ERR188273_depth.tsv
## chrX 21649 1
## chrX 21650 1
## chrX 21651 1
## chrX 21652 1
## chrX 21653 1
## chrX 21654 1
## chrX 21655 1
## chrX 21656 1
## chrX 21657 1
## chrX 21658 1
The average depth can be calculated by summing the third column and
dividing by the total number of bases (be sure to use -a with
samtools depth as that will output all positions including zero
depth).
samtools depth -@ 4 -a eg/ERR188273_chrX.bam | perl -ane '$t += $F[2]; END {$cov = $t / $.; printf "Bases covered:\t%.3f\nCoverage:\t%.3f\n", $., $cov}'
## Bases covered: 156040895.000
## Coverage: 0.532
The samtools mpileup command also provides depth information (but not
for reads that have a mapping quality of 0, by default) with some
additional information:
- Sequence name
- 1-based coordinate
- Reference base (when used with
-f) - Number of reads covering this position
- Read bases
- Base qualities
- Alignment mapping qualities (when used with
-s)
samtools mpileup -f genome/chrX.fa -s eg/ERR188273_chrX.bam > ERR188273_mpileup.tsv
head ERR188273_mpileup.tsv
## [mpileup] 1 samples in 1 input files
## chrX 251271 g 1 ^]. @ ]
## chrX 251272 a 1 . @ ]
## chrX 251273 a 1 . < ]
## chrX 251274 a 1 . D ]
## chrX 251275 a 1 . D ]
## chrX 251276 a 1 . D ]
## chrX 251277 t 1 . D ]
## chrX 251278 g 1 . D ]
## chrX 251279 g 1 . F ]
## chrX 251280 g 1 . B ]
Note that the start of the samtools mpileup output differ from the
start of the samtools depth output. This is because mpileup performs
some filtering by default. In the case of this example, read pairs that
are not both mapped will be ignored. To count these “orphan” reads, use
the --count-orphans argument.
samtools mpileup -f genome/chrX.fa --count-orphans -s eg/ERR188273_chrX.bam > ERR188273_mpileup_orphans.tsv
head ERR188273_mpileup_orphans.tsv
## [mpileup] 1 samples in 1 input files
## chrX 21649 g 0 * * *
## chrX 21650 a 1 . D !
## chrX 21651 t 1 . F !
## chrX 21652 c 1 . F !
## chrX 21653 a 1 . H !
## chrX 21654 c 1 . G !
## chrX 21655 g 1 . H !
## chrX 21656 a 1 . B !
## chrX 21657 g 1 . H !
## chrX 21658 g 1 . I !
In addition mpileup performs “per-Base Alignment Quality” (BAQ) by
default and will adjust base quality scores. The default behaviour to to
skip bases with baseQ/BAQ smaller than 13. If you are finding
discrepancies between mpileup’s coverage calculation with another
coverage tool, you can either set --min-BQ to 0 or use --no-BAQ to
disable BAQ.
I have an old blog
post on using
mpileup.
samtools coverage will provide the following coverage statistics:
rname- Reference name / chromosomestartpos- Start positionendpos- End position (or sequence length)numreads- Number reads aligned to the region (after filtering)covbases- Number of covered bases with depth >= 1coverage- Proportion of covered bases [0..1]meandepth- Mean depth of coveragemeanbaseq- Mean base quality in covered regionmeanmapq- Mean mapping quality of selected reads
samtools coverage eg/ERR188273_chrX.bam
## #rname startpos endpos numreads covbases coverage meandepth meanbaseq meanmapq
## chrX 1 156040895 1110685 3402037 2.18022 0.532299 36.3 59.4
The example BAM file only contains reads for chrX hence the statistics
are only returned for chrX.
Returning to our coverage definition at the start of this section:
- average depth of each covered base =
meandepth - percentage of bases covered =
covbases
The mosdepth tool can also
calculate depth (and much faster than samtools depth) per base or
within a given window. The output is given in a BED file, where the
fourth column indicates the coverage.
mosdepth ERR188273 eg/ERR188273_chrX.bam
gunzip -c ERR188273.per-base.bed.gz | head
## chrX 0 21648 0
## chrX 21648 21718 1
## chrX 21718 251270 0
## chrX 251270 251391 1
## chrX 251391 265950 0
## chrX 265950 266021 1
## chrX 266021 266096 2
## chrX 266096 269848 0
## chrX 269848 269923 1
## chrX 269923 270095 0
mosdepth coverage.
cat ERR188273.mosdepth.summary.txt
## chrom length bases mean min max
## chrX 156040895 76303957 0.49 0 40804
## total 156040895 76303957 0.49 0 40804
Coverage in using a 500 bp window.
mosdepth -n --fast-mode --by 500 ERR188273_500 eg/ERR188273_chrX.bam
gunzip -c ERR188273_500.regions.bed.gz | head
## chrX 0 500 0.00
## chrX 500 1000 0.00
## chrX 1000 1500 0.00
## chrX 1500 2000 0.00
## chrX 2000 2500 0.00
## chrX 2500 3000 0.00
## chrX 3000 3500 0.00
## chrX 3500 4000 0.00
## chrX 4000 4500 0.00
## chrX 4500 5000 0.00